Microfluidic Immunoassay Device for Blood Analysis
KEY INFORMATION
Healthcare - Medical Devices
TECHNOLOGY OVERVIEW
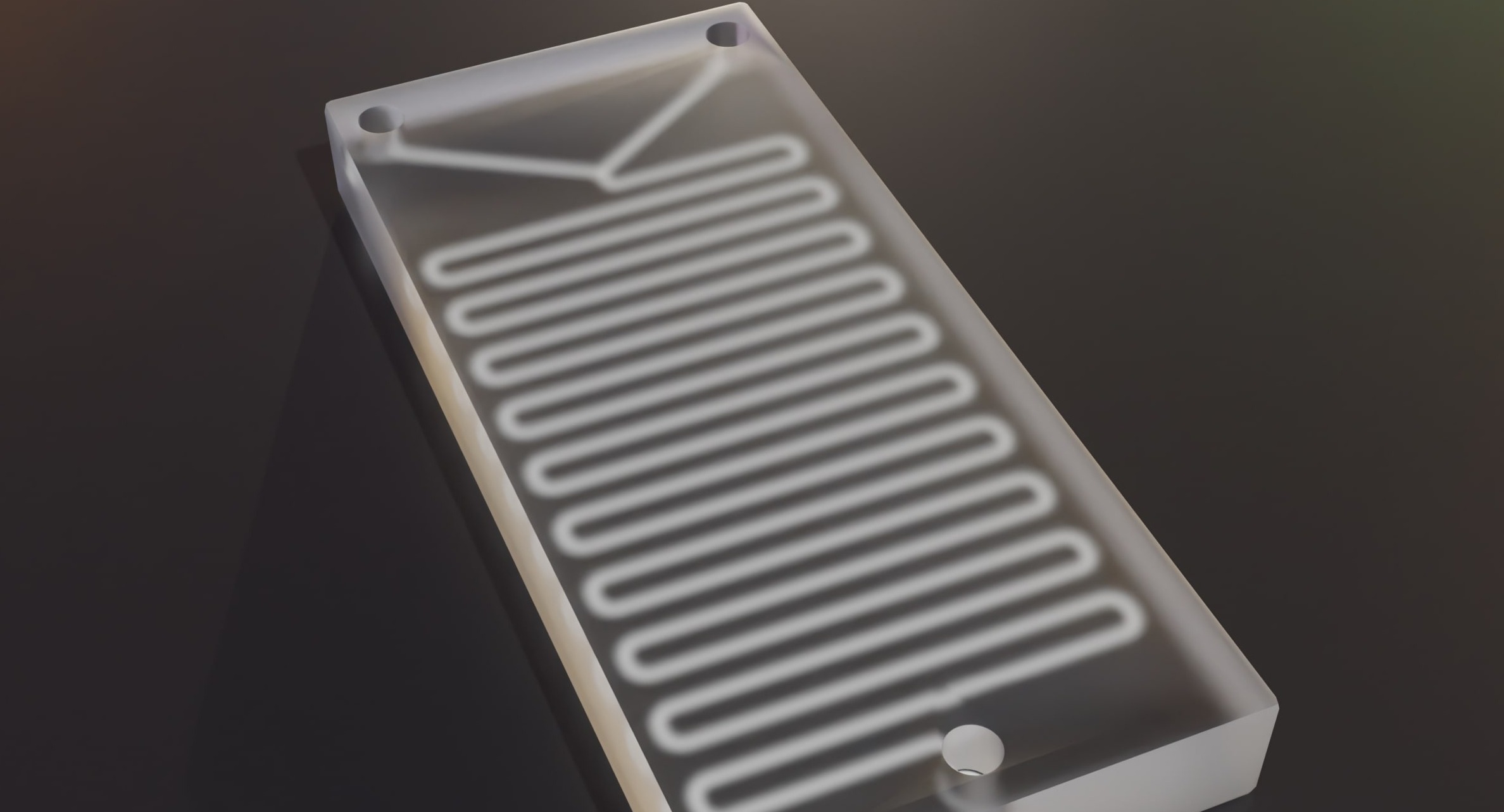
A microfluidic chip-based mechanism has been developed as a Point-of-Care Testing (POCT) device to replace Lateral Flow Assays (LFA) for fast and convenient blood analysis. The microchip system utilises the principle of immunoassays but with high accuracy and compatibility to different signalling tags, providing a quantitative readout. Conventional immunoassays involve multistep procedure and long process time. While LFAs are fast and convenient, they are qualitative. The device demonstrated a one-step assay that can achieve equal or higher sensitivities than standard methods within significantly shorter total processing time. In a microfluidic device, the sample flows in precisely defined microchannels, which allow better control of fluid behaviour and higher consistency in testing results compared to LFA in which the sample flows by wicking through the porous paper-based material. This technology resides in the assembly of components and materials to immobilise antibodies or antigens onto the chip which can be easily scaled for commercial production.
The technology owner is seeking collaborations with manufacturers of IVD devices or Medtech companies to out-license the technology and expand the range of antibodies targets for the microchip.
TECHNOLOGY FEATURES & SPECIFICATIONS
The core technologies of the invention include:
- Methods to prepare nitrocellulose substrate for antibody immobilization. The materials, fabrication methods and reagent integration techniques are readily compatible with high-volume manufacturing, allowing the prototype to have high potential for commercialization.
- Methods to prepare and storage of dried reagent on chip. The shelf-life of the dried reagents is around 3 months.
- The Limit of Detection (LOD) is 0.1 ng/mL with a total process time of 15 minutes.
- No washing steps required.
- The device is compatible with all antibodies and antigen immobilisation.
- Signalling antibodies can be fluorescence or colorimetric which can be easily paired with off-the-shelf detector.
- Any biomarkers that can be analysed by LFA can be used on this platform.
POTENTIAL APPLICATIONS
The device can be used in medical, veterinary and other related industries for diagnosis or screening purposes.
Unique Value Proposition
The device provides fast and accurate method for detecting biomarkers in blood. The device has been used to measure the blood concentration of Anti-Mullerian Hormone (AMH), which is an indicator for women fertility (a high AMH levels is more likely to achieve a successful pregnancy than low levels). Some examples of LFA that can be transferred to the microfluidic platform includes HCG pregnancy test, Covid ART, AMH fertility test etc. The significance for the microfluidic device is the accuracy and reliability of the results for quantitative analysis.
